Importance Although prior studies have suggested a role of cardiometabolic health on pathogenesis of amyotrophic lateral sclerosis (ALS), the association with diabetes mellitus has not been widely examined. Amyotrophic lateral sclerosis is the most common motor neuron disorder. Several vascular risk factors have been associated with decreased risk for ALS. Although diabetes is also a risk factor for vascular disease, the few studies of diabetes and ALS have been inconsistent.
Objective To examine the association between diabetes and obesity, each identified through International Statistical Classification of Diseases, Eighth or Tenth Revision codes in a hospital registry, and ALS using data from the Danish National Registers.
Bạn đang xem: Diabetes Mellitus, Obesity, and Diagnosis of Amyotrophic Lateral Sclerosis: A Population-Based Study
Design, Setting, and Participants Population-based nested case-control study of 3650 Danish residents diagnosed as having ALS between January 1, 1982, and December 31, 2009, and 365 000 controls (100 for each ALS case) matched on age and sex. The analysis was conducted in September and October 2014.
Main Outcomes and Measures Adjusted odds ratio for ALS associated with diabetes or obesity diagnoses at least 3 years prior to the ALS diagnosis date.
Results When considering diabetes and our obesity indicator together, the estimated odds ratio for ALS was 0.61 (95% CI, 0.46-0.80) for diabetes and 0.81 (95% CI, 0.57-1.16) for obesity. We observed no effect modification on the association with diabetes by sex. We did find a significant modification by age at ALS diagnosis and age at first mention of diabetes in the hospital registers. The protective association was stronger with increasing age at ALS diagnosis (P = .01), and the odds ratio for first mention of diabetes was 1.66 (95% CI, 0.85-3.21) before age 40 years but 0.52 (95% CI, 0.39-0.70) for older ages. These results are consistent with different associations for type 1 vs type 2 diabetes.
Conclusions and Relevance In this Danish nationwide study to investigate the association between diabetes and ALS diagnosis, our findings are in agreement with previous reports of a protective association between vascular risk factors and ALS and suggest that type 2 diabetes, but not type 1, is protective for ALS.
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive degeneration of the upper and lower motor neurons,1 with approximately half of the patients dying within 3 years of onset.2 The ALS incidence rate is between 1.5 and 2.5 per 100 000 persons per year3 and increases with increasing age, although incidence appears to decrease after age 80 years.4 Although a recent study found ALS heritability to be higher than previously reported,5 the etiology of ALS pathogenesis is not completely understood, with environmental contributions likely contributing to the disease.2 Because of the rarity of the disease, large-scale cohort studies are difficult and so have not commonly been used to investigate potential risk factors, but the need for more such studies has been recognized.6
Recent reports have found a protective association between vascular risk factors, such as obesity or higher body mass index (BMI; calculated as weight in kilograms divided by height in meters squared),7 higher cholesterol levels8 and hyperlipidemia,9,10 and ALS incidence as well as ALS survival.11-14 These findings, coupled with reports that energy metabolism and homeostasis are also involved in ALS pathogenesis,9 suggest that the pathophysiology of ALS is multifactorial.15 Patients with type 2 diabetes mellitus have, on average, higher BMI, elevated blood lipid levels,16 and defective energy metabolism.17 Moreover, diabetes is a metabolic disorder with increasing prevalence globally.18 However, the association between diabetes and ALS has not been widely explored. Recently, a case-control study in the Netherlands reported a protective, albeit insignificant, association between diabetes and ALS onset.8
To assess the potential link between diabetes and risk of ALS, we examined the association between hospital admissions for diabetes and ALS diagnosis between January 1, 1982, and December 31, 2009, in the entire Danish population. We used data obtained from the Danish National Registers system, through which details of all Danish residents can be linked.19
Data were obtained from the Danish National Registers system, through which details on demographic characteristics and certain health outcomes of all approximately 6 million Danish residents can be linked based on a 10-digit unique personal identifier. The Danish National Patient Register (NPR) was established in 1977 and is a comprehensive patient register, including nationwide clinical and administrative records for all somatic inpatient data. Outpatient data have also been included in the NPR since 199520 and are considered in our analyses. The ALS cases in this study were diagnosed between January 1, 1982, and December 31, 2009. The analysis was conducted in September and October 2014. This study was determined to be exempt by the Harvard School of Public Health Institutional Review Board and was approved by the Danish Data Protection Agency. Participants in our analyses were not required to provide informed consent; by Danish legislation, participants are not required to provide informed consent when no biological samples are obtained.
We identified ALS cases based on their International Classification of Diseases (ICD) discharge diagnoses, ie, ICD-8 code 348.0 (ALS) until 1993 and ICD-10 code G12.2 (motor neuron disease) thereafter. As the diagnosis date (index date), we used the date of the first relevant code. We only included patients who were at least 20 years old when diagnosed and we restricted analyses to cases identified after 1982 to avoid inclusion of prevalent cases in the early years of the NPR. In a validation substudy, we obtained 173 medical records for ALS cases identified through the NPR. Of these, only 13 had no ALS (7.5%). The estimated positive predictive value, including the clinically suspected ALS cases, was 0.93 (95% CI, 0.88-0.96).21
Controls were selected through the Danish Civil Registration System, which was established in 1968 and includes administrative records (eg, date and place of birth, vital status, and history of civil status and addresses) on all persons living in Denmark, and records are kept even when a person dies or emigrates.19 We selected 100 controls for each identified case, alive at the time of the matched case’s ALS diagnosis (the index date) and individually matched on sex and year of birth.
Diabetes is a disease caused by either insulin deficiency or a decreased responsiveness to insulin. Type 1 diabetes (formerly called juvenile or insulin-dependent diabetes), in most cases of autoimmune etiology, is associated with complete or almost complete insulin absence. Conversely, type 2 diabetes (non-insulin-dependent diabetes) is characterized by insulin resistance and occurs later in life as a syndrome of mainly overweight adults.22 We identified diabetes cases based on hospital discharge ICD codes for type 1 and type 2 diabetes in the NPR starting in 1977. Specifically, we used ICD-8 codes 249 and 250, respectively, and ICD-10 codes E10 and E11, respectively. Identification of diabetic patients using hospital data has been found to be highly reliable,23 but misclassification between type 1 and 2 diabetes is likely24 and a complete classification by type is not possible.25 This is also borne out in our own data, in which we found 74% of those with type 1 diabetes to also have a code for type 2. We therefore did not conduct separate analyses for type 1 and type 2 diabetes as distinguished by the ICD codes.
Our main analysis only considered diabetes discharges that occurred at least 3 years prior to the index date to minimize the chance that any observed association with diabetes could be due to the effects of underlying but not yet diagnosed ALS. To assess the robustness of our results, we repeated our analyses using only diabetes discharges that occurred at least 5 and 7 years prior to the index date.
We used hospital discharge data to identify patients diagnosed as having obesity and lipid metabolism disorders. Specifically, we considered obesity admissions using ICD-8 code 277.99 and ICD-10 codes E65.0 to E66.9.26 We refer to this throughout the article as obesity, but it should be noted that this is not based on a BMI greater than 30, the typical definition of obesity, and most likely reflects a much more elevated BMI. We also obtained hyperlipidemia and hypercholesterolemia data from the NPR (ICD-8 code 279.00 and ICD-10 codes E78.0-E78.5). As for diabetes, our main analyses used discharges that occurred at least 3 years prior to the index date.
We additionally considered marital status (nonmarried, married, divorced, and widowed), residence region at the index date (Copenhagen, ie, the capital, Copenhagen suburbs, other large cities, provincial towns, rural areas, Greenland, and unknown), and socioeconomic status (SES) in our analyses. We coded SES as the highest level attained by either the participant or his or her spouse based on his or her job title. Job titles were categorized in 5 groups according to the Danish Institute of Social Sciences, which reasonably captures SES variation in Denmark.27 Group 1 includes corporate managers and participants in academia; group 2, proprietors, managers of small businesses, and teachers; group 3, technicians and nurses; group 4, skilled workers; and group 5, unskilled workers. We included a separate group for participants with unknown job titles.
The NPR does not include data on potential individual-level confounders such as smoking. We therefore used chronic obstructive pulmonary disease (COPD) codes (ICD-8 codes 490-493 and 518 and ICD-10 codes J40-J47) at least 1 year prior to the index date as an indicator for smoking. In developed countries, up to 70% of COPD cases are among tobacco smokers,28 and COPD is thus recognized as an indicator for smoking.
Xem thêm : 2015-2021 SUBARU WRX STI VLAND PRE-BUILT HEADLIGHTS
For both diabetes and obesity, we ran conditional logistic regressions with strata defined by the 1:100 case-control sets adjusting for SES. We also ran models including COPD, marital status, and region of residence at the index date as covariates. Finally, we considered obesity and diabetes together in a model and assessed the association with diabetes in models stratified by obesity levels.
We also assessed potential effect modification by sex and age. For the effect modification, we included an interaction term between our exposure variable (diabetes or obesity) and sex or age in separate models, adjusting for SES, and examined the statistical significance of the interaction term at the α = .05 level.
Because misclassification in hospital diagnoses for type 1 and 2 diabetes is likely,24,25 in an effort to separate potentially different effects, we attempted to separate types of diabetes based on the age at first mention of diabetes in the hospital registers. Those with diabetes onset at younger ages are more likely to have type 1 diabetes. We therefore conducted analyses considering separately diabetes with early and late first hospital discharge codes. We used a lower cutoff of age 40 years (the decade closest to but lower than the first decile of the age distribution at first diabetes-related admission) and then also considered different cutoffs at older ages in 5-year increments.
All statistical analyses were conducted using R statistical software version 3.0.3 (R Foundation for Statistical Computing).
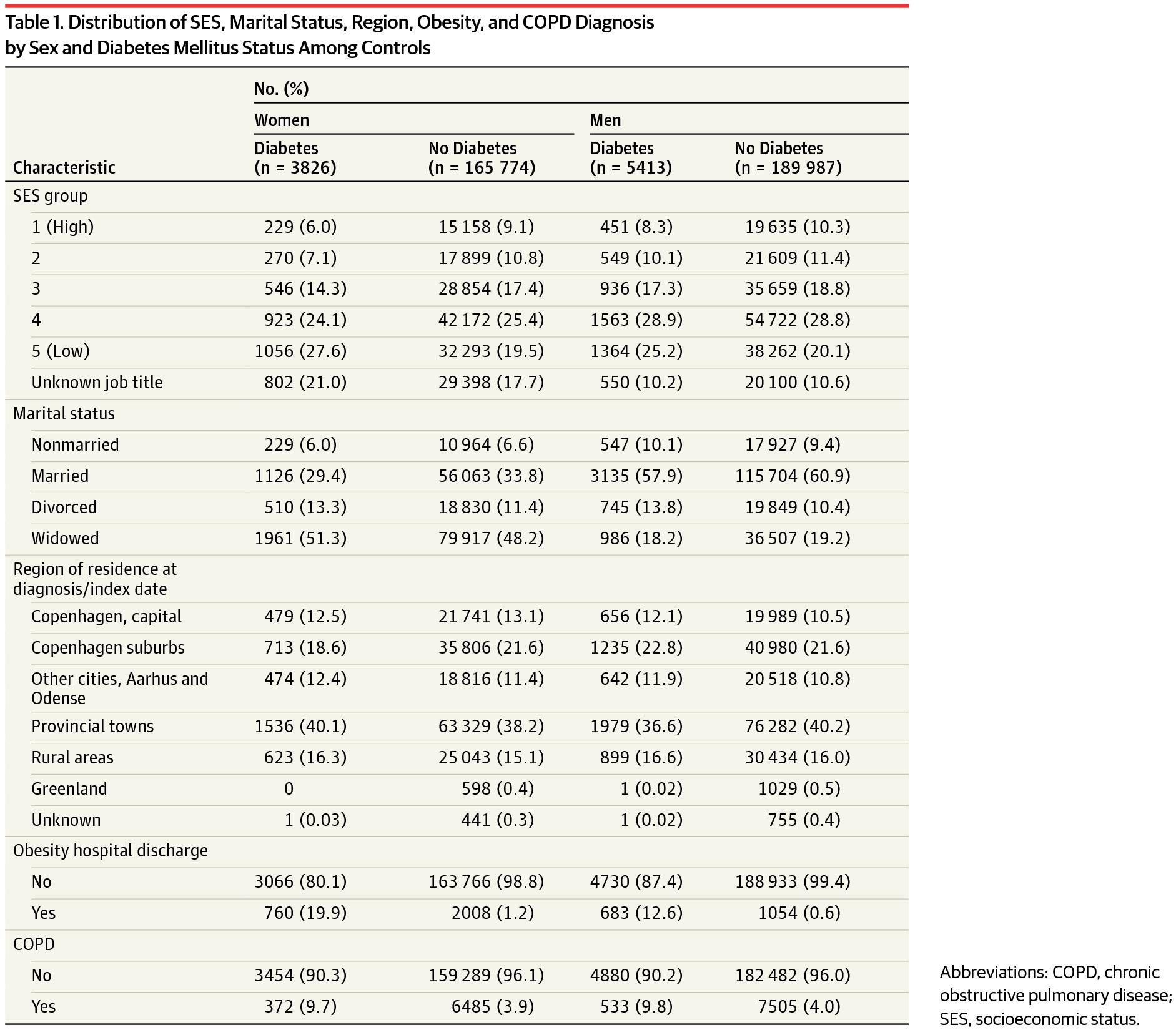
We identified 3650 ALS cases in the NPR between January 1, 1982, and December 31, 2009, that satisfied our inclusion criteria. The mean (SD) age at diagnosis was 65.4 (11.6) years and 46.5% of all cases were female. The distributions of the SES groups, marital status, region of residence, obesity, and COPD status are presented in Table 1 by sex and diabetes status.
Using NPR records, we identified 9294 participants with diabetes at least 3 years prior to the index date (date of ALS diagnosis or the same date for the matched controls), 55 of whom were subsequently diagnosed as having ALS. The mean (SD) age at the first diabetes-related diagnosis was 59.7 (12.1) years and there was no significant difference between cases and controls (P = .07). Among patients with ALS who had diabetes, the first diabetes-related admission occurred a mean (SD) of 9.8 (6.1) years before the ALS diagnosis.
We observed a strong protective association between prior diabetes-related admissions and ALS (Table 2); the crude odds ratio (OR) was 0.59 (95% CI, 0.45-0.77). Adjusting for SES, marital status, region of residence, and prior COPD admissions did not change the effect estimates, nor did adjusting for our obesity indicator (OR = 0.61; 95% CI, 0.46-0.80). The ORs were similar when excluding diabetes in the 5 or 7 years before the index date (OR = 0.66; 95% CI, 0.50-0.88; and OR = 0.69; 95% CI, 0.50-0.96, respectively).
We identified 4536 participants with a hospital record for obesity at least 3 years prior to the index date. Effect estimates for obesity in unadjusted models or adjusting for SES, COPD status, marital status, and residence at diagnosis were similar to those for diabetes, but when diabetes was included in the model the association was weakened and was no longer significant (OR = 0.81; 95% CI, 0.57-1.16) (Table 2). Further, we observed no effect modification by diabetes (P = .99). We identified 4168 participants with a discharge for either hyperlipidemia or hypercholesterolemia. We observed no association between these and ALS.
We found no evidence for effect modification by sex of either the diabetes association (P = .84) or the obesity association (P = .72). We also found no effect modification by age at ALS diagnosis of the association with obesity (P = .42). Conversely, a significant effect modification by age at ALS diagnosis on the association between diabetes and ALS was observed (P = .01), with lower effect estimates observed at older ages. Specifically, the effect of diabetes on ALS was increased until age 51 years (eg, at ALS diagnosis at age 35 years, OR = 1.68; 95% CI, 0.75-3.78), although this increase did not reach statistical significance. The effect estimates became protective at older ages (statistically significant at ages ≥61 years, eg, at 65 years, OR = 0.65; 95% CI, 0.50-0.85) (Figure 1).
We also found a significant effect modification by age at first diabetes diagnosis, as a proxy to separate between type 1 and type 2 diabetes (Figure 2). The distribution of age at first diabetes-related diagnosis is presented in the eFigure in the Supplement. When the cutoff for age at diabetes diagnosis was 40 years, the difference in effect estimates for diabetes among those older and younger than the cutoff was significant (P = .002), with a significantly protective association among participants older than 40 years at first diabetes-related diagnosis (OR = 0.52; 95% CI, 0.39-0.70) and harmful effects for participants younger than 40 years (OR = 1.66; 95% CI, 0.85-3.21), although the increased OR did not reach statistical significance (P = .14). For all examined cutoffs, the associations in the category of older age at diabetes diagnosis remained robust and similar to that in the main analysis. With decreasing cutoff age, however, we observed an upward trend in the association with diabetes among those in the younger group.
We conducted a retrospective population-based study to examine the association between diabetes- and obesity-related hospital admissions and risk of ALS diagnosis. When including both in the same model, we observed a significantly protective association with diabetes, but not obesity, on risk of ALS. As we noted, however, a hospital discharge code for obesity likely reflects much more severe obesity than the typical definition of obesity as a BMI greater than 30. The association with diabetes was modified by both age at ALS diagnosis and age at diabetes diagnosis, with older age at diagnosis for either disease associated with lower risk for ALS. Both earlier age at ALS diagnosis and earlier age at first diabetes mention in the hospital registers increase the likelihood that the diabetes is type 1. These results thus may suggest that the protective association is with type 2 diabetes but not type 1, which may have the opposite association, ie, an increased risk of ALS.
The association between diabetes and ALS has not been widely examined, with inconsistent findings initially.29 A recent case-control study in the Netherlands reported a protective association that did not reach statistical significance; misclassification due to self-reported diabetes could have contributed to the attenuated association.8 Our results, however, are consistent with a recent study in Sweden that reported an OR of 0.66 (95% CI, 0.45-0.80) for diabetes at least 3 years prior to ALS diagnosis.30
Previous studies found a protective association between vascular risk factors and ALS and found that obesity was uncommon among patients with ALS.31 Scarmeas et al32 found that individuals who had always been slim were at a significantly higher risk for ALS. O’Reilly et al7 reported that ALS rates were significantly lower among obese individuals (relative rate = 0.73; 95% CI, 0.55-0.96). While the reasons for this association remain unclear, the protective association between higher BMI and ALS is consistently observed,7,32,33 and this has been suggested to be related to hypermetabolism that is also often observed with ALS.9,15 Hyperlipidemia and elevated cholesterol levels have also been associated with lower ALS risk.8,10,31
The underlying pathophysiological mechanisms of ALS are likely multifactorial, with defective energy metabolism and homeostasis likely playing an important role in pathogenesis.9,15 Overweight and obese people are at higher risk for type 2 diabetes,34 and those with type 2 diabetes are at higher risk for elevated cholesterol levels, hyperlipidemia, and vascular disease.35 Our findings are thus consistent with prior reports suggesting an inverse association between cardiometabolic health and ALS. In addition, higher levels of physical activity have been associated with increased risk of ALS32 and are also generally associated with lower BMI, lower risk of diabetes, and generally better cardiometabolic health. It is possible that associations with BMI and diabetes are simply the result of their own association with physical activity. On the other hand, effects of physical activity on cardiometabolic health in general could be the reason for the association between physical activity and ALS. Studies with detailed data on all of these factors, including relevant timing, will be critical for sorting out the relationships between these variables and risk of ALS. We also cannot rule out the possibility that genetic risk factors that give rise to ALS are protective for cardiometabolic disorders and, specifically, diabetes.
If the protective association with diabetes results from some causal association with an aspect of diabetes rather than as a result of correlation with something else, then several possibilities could be surmised. Diabetes medications could be protective. A limited literature on a few compounds to date does not suggest such an effect,36,37 but not all diabetic medications have been examined. Excitotoxicity or uric acid could also play a role. Glutamate excitotoxicity, a consequence of extracellular glutamate accumulation,38 has been implicated in ALS pathogenesis.39,40 The hyperglycemia that is characteristic of individuals with untreated type 2 diabetes35 (only 14%-17% of individuals with type 2 diabetes are treated with insulin41), through enhancing glutamate uptake,42 could protect against excitotoxic effects. Diabetes has also been associated with high concentrations of uric acid,43 which has been associated with reduced incidence of other neurodegenerative diseases44,45 and prolonged survival in ALS.46
We found that the association between diabetes and ALS varied by age at ALS diagnosis, trending toward a harmful association with earlier age at ALS diagnosis. This could reflect different associations between type 1 vs type 2 diabetes and ALS because earlier age at ALS onset would also imply earlier age at diabetes onset prior to ALS, which would therefore be more likely to be type 1 diabetes. However, ALS at earlier ages is likely more commonly genetic in origin; for example, the presence of C9orf72 is more common among younger patients with ALS.47-49 Therefore, the variation by age at ALS diagnosis could also suggest a different relation between diabetes and ALS when the ALS has a stronger genetic influence. We also found variation in the association with diabetes by the age at diabetes diagnosis. This more strongly suggests a possible difference in the relation of type 1 and type 2 diabetes with ALS because it did not restrict the age at ALS diagnosis. The trend toward an increased risk of ALS following type 1 diabetes is consistent with previous findings,30,50 but more conclusive evidence will require data with more certain determination of diabetes type than what is available in the NPR.
An opposite association between type 1 diabetes and ALS could be expected based on uric acid or blood glucose levels. In contrast to those with type 2 diabetes, those with type 1 diabetes have lower uric acid levels51 and are more prone to hypoglycemic excursions because they are all medicated and at risk for insulin treatment-induced hypoglycemia.52,53 Furthermore, most individuals with type 1 diabetes have type 1A diabetes, ie, of autoimmune etiology,35,53 and are at higher risk for presenting with additional autoimmune disorders.54 Autoimmune mechanisms have also been implicated in ALS.50,55
Neither outcome nor exposure misclassification can be excluded from our analysis. However, both ALS21 and diabetes-related23 hospital discharges have been found to be highly reliable. Furthermore, using hospital diagnoses, we are likely only capturing the most severe cases of diabetes, obesity, and disorders of lipid metabolism. Diabetic individuals have reduced survival compared with nondiabetic individuals, so the possibility that survival differences could affect our results must be considered. However, if this was biasing our findings, we would expect to see stronger bias with diabetes that occurred earlier relative to ALS. Analyses restricting to diabetes at least 5 or 7 years prior to ALS showed results similar to or slightly higher than diabetes at least 3 years prior to ALS.
Xem thêm : What’s up with Subaru lug studs?
An additional limitation of our study is the lack of individual-level information on lifestyle factors such as smoking. In Denmark, smoking has been shown to be very highly correlated with occupational status56; thus, adjusting for occupation-based SES would partially also adjust for smoking status. More importantly, however, while the association between smoking and ALS is not completely consistent,57 it appears associated with an increased risk of ALS. Because smoking is a risk factor for diabetes, this would not account for a protective association.
We conducted a nationwide, population-based study and observed an overall protective association between diabetes and ALS diagnosis, with the suggestion that type 2 diabetes is protective and type 1 diabetes is a risk factor. Although the mechanisms underlying this association remain unclear, our findings focus further attention on the role of energy metabolism in ALS pathogenesis.
Corresponding Author: Marianthi-Anna Kioumourtzoglou, ScD, Department of Environmental Health, Harvard T. H. Chan School of Public Health, 401 Park Dr, Landmark Center, Third Floor E, Boston, MA 02215 (marianthi.anna@mail.harvard.edu).
Accepted for Publication: April 8, 2015.
Published Online: June 1, 2015. doi:10.1001/jamaneurol.2015.0910.
Author Contributions: Dr Weisskopf had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Kioumourtzoglou, Gredal, Hansen, Weisskopf.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Kioumourtzoglou, Rotem, Weisskopf.
Critical revision of the manuscript for important intellectual content: Seals, Gredal, Hansen, Weisskopf.
Statistical analysis: Kioumourtzoglou, Seals, Weisskopf.
Obtained funding: Hansen, Weisskopf.
Administrative, technical, or material support: Kioumourtzoglou, Gredal, Hansen.
Study supervision: Weisskopf.
Conflict of Interest Disclosures: None reported.
Funding/Support: This work was supported by grant 5R01 ES019188-02 from the National Institute of Environmental Health Sciences. Dr Kioumourtzoglou and Mr Seals are supported in part by training grant T32 ES007069 from the National Institutes of Health. Mr Seals is also supported in part by the Taplin Fellowship.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: Alessandro Doria, MD, PhD, Department of Epidemiology, Harvard T. H. Chan School of Public Health and Joslin Diabetes Center, Harvard Medical School, Boston, Massachusetts, provided critical review of an earlier version of the manuscript; he received no compensation.
Nguồn: https://vuihoctienghan.edu.vn
Danh mục: Info
This post was last modified on Tháng mười một 28, 2024 5:34 chiều