Objective To compare the analgesic efficacy of oral tramadol hydrochloride and oral diclofenac sodium for posttonsillectomy pain management.
Design Single-blind (surgeon and research team members), prospective, randomized, controlled clinical trial.
Bạn đang xem: Tramadol vs Diclofenac for Posttonsillectomy Analgesia
Patients and Methods Sixty-four patients 11 years and older undergoing bipolar electrocautery tonsillectomy were randomized to either the oral tramadol or the oral diclofenac postoperative pain group. Patients recorded pain levels twice daily for 14 days using a visual analogue scale.
Results Pain scores for the 14 days were not significantly different between the oral tramadol and oral diclofenac groups. There were no significant differences in the incidence of postoperative hemorrhage and hospital readmission for uncontrolled pain.
Conclusion Oral tramadol can deliver the same analgesic efficacy as oral diclofenac for posttonsillectomy pain relief, which might be beneficial for avoiding the adverse effects of nonsteroidal anti-inflammatory drug therapy.
TONSILLECTOMY is a common operation performed by otolaryngologists. Various surgical techniques and adjunctive treatments are used.1 Pain is the most significant obstacle to the rehabilitation of a patient after tonsillectomy, and it can affect analgesic consumption, duration of inpatient care, oral intake, and return to regular function.2 Several factors can affect the duration and intensity of posttonsillectomy pain, including individual patient perceptions of pain, surgical technique, pretonsillectomy injection of peritonsillar local anesthetic, peritonsillectomy analgesia, posttonsillectomy analgesia, mouth rinses, gargles and sprays, diet and fluid intake, and antibiotics and corticosteroids.1 The specific factor of interest to us is posttonsillectomy analgesia, defined as analgesia from day 1 onward as distinguished from peritonsillectomy analgesia used on the day of tonsillectomy (day 0). Ideally, an analgesic for posttonsillectomy pain management should be available in an oral formulation with high potency, be nonrespiratory depressing, and have a favorable adverse effect profile.
Tramadol hydrochloride is a centrally acting analgesic agent introduced into the United Kingdom in 1994, the United States in 1995, and New Zealand in 1998. It was first synthesized in 1968, and Germany has had an injectable formulation available since 1977 and an oral formulation since the early 1980s. Tramadol has been used clinically and evaluated during the past 20 years, with broad indications leading to its widespread use.3,4 Use of tramadol for posttonsillectomy pain management has been reported in the literature in only 3 studies, to our knowledge. One study5 on posttonsillectomy analgesia compared diclofenac sodium monotherapy with tramadol hydrochloride retard and naproxen combined therapy, and the latter proved to be superior; the other 2 studies6,7 on peritonsillectomy analgesia used intravenous tramadol.
Therefore, the aim of this study was to compare the analgesic efficacy of oral tramadol (Tramal; CSL Pharmaceuticals, Auckland, New Zealand) and oral diclofenac (Voltaren; Novartis, Auckland) for posttonsillectomy pain management in patients 11 years and older.
Patients presenting for tonsillectomy were given a full verbal and written explanation of the study, were invited to participate, and then were included after giving informed consent. The criteria for exclusion were age younger than 11 years; elective tonsillectomy for reasons other than recurrent or chronic tonsillitis; asthma; pregnancy; breastfeeding; epilepsy; relevant drug allergies; any additional procedure excluding adenoidectomy, ie, uvulectomy; and expression of a strong desire to receive only one of the posttonsillectomy analgesics used in the trial.
Xem thêm : ‘This can’t be right’: Customers are just now finding out how many calories are in a Four Loko
Patients were admitted to the hospital through the day-surgery unit or inpatient ward. Consecutive patients were randomized in an unblocked fashion using a random number generator (Excel; Microsoft Corp, Redmond, Wash) to form 2 groups. One group received a 14-day supply of oral tramadol and took 100 mg in the morning, 50 mg in the afternoon, and 50 mg in the evening or, if the patient weighed less than 50 kg, 50 mg in the morning, afternoon, and evening. The other group received a 14-day supply of oral diclofenac and took 50 mg in the morning, afternoon, and evening or, if the patient weighed less than 50 kg, 50 mg in the morning and evening. Patients were instructed verbally and via the trial drug packet to use the medication 3 times per day, at approximately 8 AM, 2 PM, and 7 PM to allow recording of the pain score 2 hours after receiving the dose. In addition, both groups received acetaminophen elixir or tablets, 15 mg/kg per dose for up to 5 doses per day for up to 14 days; benzydamine hydrochloride spray (Difflam; 3M Pharmaceuticals, Auckland), 2 sprays up to a frequency of every 2 hours; and amoxicillin syrup, 20 mg/kg per day in 3 divided doses for 7 days. The 4 prescribed medications were prepared and packaged by a clinical pharmacist. Preoperative preparation, anesthesia induction, and maintenance were ordered as per the Department of Anesthesia, Palmerston North Hospital, Palmerston North, New Zealand, with no routine use of premedicants and an intravenous opioid as the analgesic component of the general anesthetic.
Tonsillectomy was performed by a single masked participating surgeon (M.J.C.) by first injecting 10 mL of 0.25% bupivacaine hydrochloride with 1:200 000 epinephrine into each tonsillar bed followed by bipolar electrocautery dissection of each tonsil and fossae to achieve hemostasis.
The prepared trial medication package was given to the patient in the recovery room after tonsillectomy.
After tonsillectomy, patients scored their resting pain level on a 100-mm visual analogue scale (VAS) at 10 AM and 9 PM from day 1 to day 14. At the time of consenting for the trial and on discharge from the hospital patients were instructed on how to use the VAS, a well-accepted and validated method of pain measurement that has been used previously in the investigation of posttonsillectomy pain.8 In addition, patients recorded the number of doses taken per day for each of the 4 posttonsillectomy medications. Posttonsillectomy hemorrhage, vomiting, cyclizine hydrochloride use, and hospital readmission incidence were also recorded.
The Manawatu-Wanganui, New Zealand, ethics committee approved the study. The study was planned with 25 patients in each group to detect a large effect of 0.8 SD between the group means, with power of 80% and α2 = .05. For VAS scores at any single follow-up time, an SD of 24.5 mm was estimated from the VAS pain scores of a placebo group undergoing tonsillectomy9 by averaging the variances for the different times. Therefore, a mean difference of approximately 20 mm between the groups could be detected at any point after surgery. Revill et al10 showed that an individual could score a VAS to within ±7 mm on a 100-mm scale, so the detectable difference is well outside the random error of scoring. Because recordings are made twice daily for 14 days, much smaller differences can be detected in the averaged postoperative VAS score during this period. However, it was not possible to plan sample sizes using average scores because the variance of such average scores was not available from the literature.
Analysis was carried out using a database and statistics package (Epi-Info, version 6; Centers for Disease Control and Prevention, Atlanta, Ga). Categorical data were analyzed using χ2 tests for contingency tables; if expected numbers were too small, the Fisher exact test was used. Continuous data such as average pain scores or dosing frequency were analyzed using Mann-Whitney U or Kruskal-Wallis tests (for 2 independent groups, these nonparametric tests are merely different versions of the same test). Differences were considered statistically significant at P<.05.
Sixty-four patients were enrolled in the study, and 49 completed and returned their data collection forms. Of 15 patients who dropped out, 8 were from the tramadol group and 7 were from the diclofenac group (χ21 = 0.09, P = .77). Twelve of the 15 patients who dropped out did not return for posttonsillectomy follow-up or did not complete the posttonsillectomy data collection form, 1 discontinued the trial on day 6, 1 took both tramadol and diclofenac, and 1 was excluded from analysis because of inappropriate recording of VAS ratings. Patients who dropped out were more likely to be older (mean age, 21.1 years; P = .15) and male (χ21 = 3.61, P = .06) but of similar weight as those who completed the study (χ21 = 0.91, P = .34).
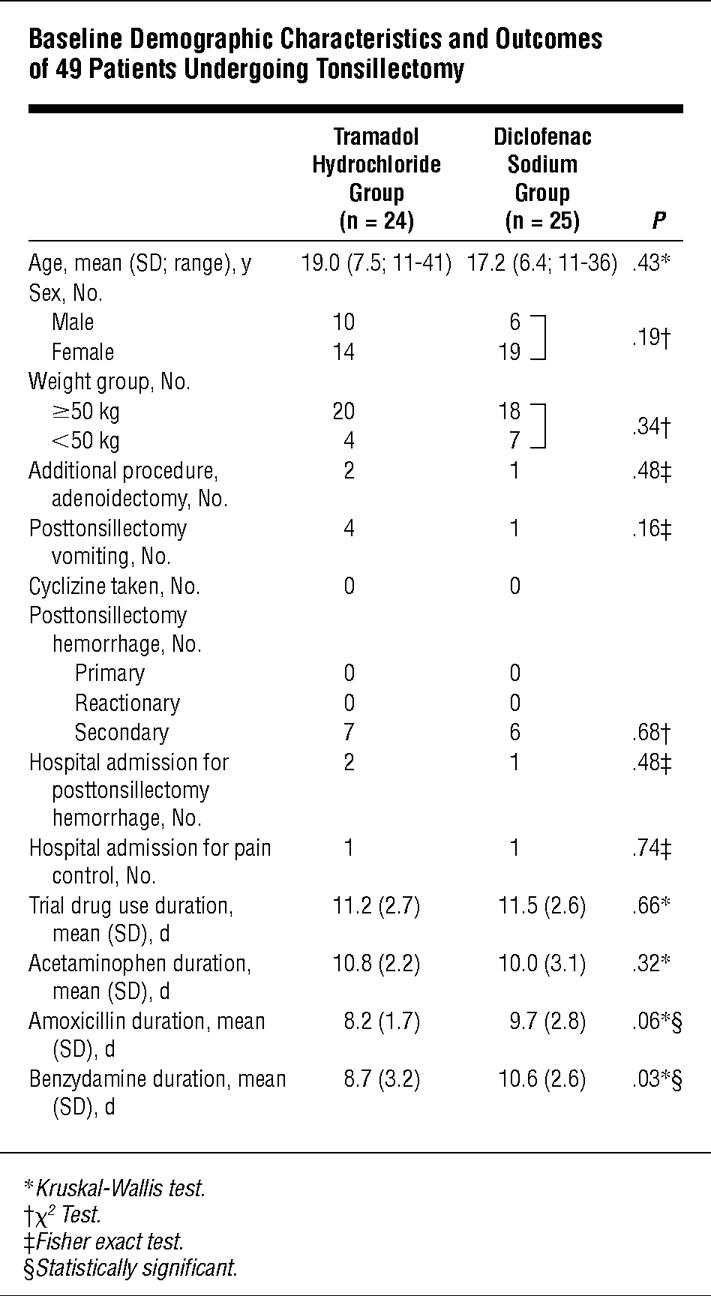
Table 1 shows results for the 49 patients who completed the study. There was no statistically significant difference between the composition of the 2 groups based on sex, age, weight group, or additional procedure. There were also no significant differences in the complications of posttonsillectomy hemorrhage incidence, cyclizine use, and hospital readmission incidence. Data on duration of medication use were 88.8% complete. There was no significant difference between the 2 groups in the duration of use of the trial drug or acetaminophen. Compared with the diclofenac group, the tramadol group used amoxicillin and benzydamine for a shorter duration. The incidence of vomiting was higher in the tramadol group vs the diclofenac group. In the excluded group, for which some data were obtained, 3 patients in the diclofenac group and none in the tramadol group reported vomiting.
Posttonsillectomy hemorrhage data were collected by questioning patients at the follow-up consultation and from emergency department presentation. Two patients in the tramadol group and 1 in the diclofenac group presented to the emergency department and were admitted to the hospital overnight for observation. At the follow-up consultation, with direct questioning it was found that another 5 patients in the tramadol group and 5 in the diclofenac group had experienced a minor hemorrhage of approximately 1 tsp usually about a week after tonsillectomy that had not concerned them enough to seek a medical consultation. No patients required operative intervention or blood transfusion. One patient in the dropout group who was taking tramadol had secondary hemorrhage on day 4 that required operative control. There were no primary or reactionary hemorrhages.
Xem thêm : Pam Dorris
Figure 1 shows that VAS pain scores recorded in the morning and evening were similar for the tramadol and diclofenac groups during the 14 days of the trial. Data were complete for 97.9% of the VAS pain scores. For analysis, missing scores were replaced by an averaged value calculated from the corresponding time of the day before and after the missing VAS score. Average VAS pain scores for the 14 days did not differ significantly (diclofenac group: mean [SD], 38.4 [17.5]; 95% confidence interval, 32.0-45.0; tramadol group: mean [SD], 37.8 [15.6]; 95% confidence interval, 32.0-43.5; P = .66, Mann-Whitney U test).
It is suggested that pain, referred otalgia, and odynophagia after tonsillectomy are caused by inflammation and its mediators, disruption of mucosa, nerve irritation, and spasm of the exposed pharyngeal muscles, with pain lasting for 10 days or longer.2,11,12 The pain can be intense—especially in adults—and because it affects posttonsillectomy rehabilitation, many attempts have been made to reduce pain.2,12
Tramadol is a centrally acting analgesic and a racemic mixture of 2 enantiomers, (+)tramadol and (−)tramadol, which have the chemical structure (1RS; 2RS)-2-[(dimethlyamino)methy1]-1-(3methoxyphenyl)-cyclohexanol hydrochloride. (+)tramadol is a mu agonist that inhibits serotonin reuptake, whereas (−)tramadol inhibits norepinephrine reuptake and increases autoreceptor activation. Tramadol has weak λ and κ effects and an oral bioavailability of 68% with the first dose and up to 90% to 100% with multiple doses. The time to onset of action is 1 hour, the time to peak concentration is 2 hours, and the elimination half-life is 6.3 hours.4 The common adverse effects are nausea, dizziness, sedation, dry mouth, sweating, and headache, all of which are generally mild, which is partly attributable to the multimodal action of the racemic, which improves efficacy without increasing the adverse effect incidence.3
Posttonsillectomy analgesia requires a nonsedating, nonrespiratory-depressing analgesic that can be used in an outpatient setting to allow early mobilization and return to regular function. Opioids do not fit easily with these requirements; they are limited by adverse effects and have monomodal action. Nonsteroidal anti-inflammatory drugs (NSAIDs) are nonsedating and nonrespiratory depressing, can be used in outpatients, and are free from the nauseagenic effects of the opioid group. These drugs act by inhibiting tissue prostaglandin production either at the site of injury or centrally, and their extensive use has confirmed that NSAIDs are effective postoperative analgesics, although contraindications and adverse effects limit use. The common and important adverse effects are impaired platelet function, peptic ulceration, renal dysfunction, and aspirin-induced asthma.13 Use of NSAIDs for posttonsillectomy pain control is controversial.13-18 Studies both support and dismiss an increase in posttonsillectomy hemorrhage in those taking NSAIDs. We know that the measured bleeding time is extended, but usually not beyond the reference range,19 an effect caused by reversible inactivation of cyclooxygenase. Long-term treatment with NSAIDs increases the risk of peptic ulceration, but no study documents an increase in gastroduodenal complications within the first week of use, although gastroduodenal mucosal erosions can be demonstrated.20 Use of NSAIDs decreases the synthesis of prostaglandins in the kidneys, which decreases renal blood flow.21 Although this effect can be accommodated in a healthy patient, in a patient with factors such as older age, a hypovolemic state, or impaired renal function, it becomes important. Aspirin-induced asthma is a condition precipitated by NSAID use that composes 5% to 10% of adult asthmatic attacks.22,23
There is a need for an alternative to NSAIDs and opioids for posttonsillectomy analgesia in patients with absolute and relative contraindications. The one previous trial5 that compared diclofenac monotherapy with tramadol and naproxen combined therapy did not exclude NSAIDs completely from either side of the comparison. That trial did not determine whether tramadol alone can provide the same analgesic efficacy as NSAIDs. In the trial reported herein, the diclofenac dose was set based on previous experience and is at the upper limit of the recommended range. For tramadol, the recommended dose for moderate pain is 50 to 100 mg 2 to 3 times daily, which is a midrange dose and the dose used in this trial. The tramadol dose can be increased to the upper limit of 400 mg/d if required, but this is not an option with diclofenac.
In conclusion, pain is a significant problem after tonsillectomy. Various options exist for the management of pain. This study demonstrates that tramadol is as effective as diclofenac for posttonsillectomy pain management.
Accepted for publication September 5, 2000.
We thank the anesthesiologists, nurses, and clinical pharmacists (especially Grant McRae) at Palmerston North Hospital who assisted with this study; and Richelle Courtney, BInfSc, information technologist, and Elisabeth Wells, PhD, biostatistician, Christchurch, New Zealand, who assisted with data analysis.
Corresponding author and reprints: Mark J. Courtney, MB, ChB, Department of Otolaryngology-Head and Neck Surgery, Wellington Hospital, Private Bag 7902, Wellington South, New Zealand (e-mail: mark.richelle@paradise.net.nz).
Nguồn: https://vuihoctienghan.edu.vn
Danh mục: Info
This post was last modified on Tháng mười một 25, 2024 4:53 chiều